The fake vaccine incident has attracted public attention. Recently, a notice from the National Medical Products Administration stated that a pharmaceutical company had serious violations of the "Good Manufacturing Practice for Pharmaceutical Products" (GMP) during the production of freeze-dried human rabies vaccines. As a result, the social anxiety of vaccines was ignited. The vaccine fraud incident once again stung the public's sensitive nerves.
How to eliminate falsified production records. Pharmaceutical companies first use the product lifecycle management (PLM) platform to ensure that drugs from R&D, pilot testing, clinical to registration applications must meet the compliance requirements of the State Food and Drug Administration (FDA); secondly, pass The Manufacturing Execution Management System (MES) system establishes a complete production process record in accordance with the Good Manufacturing Practices (GMP).
The production record of the pharmaceutical company must include: product name, specification, batch number, date and time of the start and end of production and intermediate processes, the signature of the person in charge of each production process, abnormal event reports and other data, using the real-time production collected by the MES system Record and strictly monitor the production process of medicines; the sales end of medicines should re-enable the electronic supervision code to strictly monitor the status of the production and circulation of medicines; finally, use the Internet of Things, industrial big data, and industrial cloud to ensure the development, manufacturing, and manufacturing of medicines. The entire process of quality monitoring, circulation, transportation, storage and distribution to medical institutions is under the supervision of the drug regulatory department to ensure that each box, each box, and each batch of key drug production records, operations, inventory and flow directions can be inquired in real time Happening.
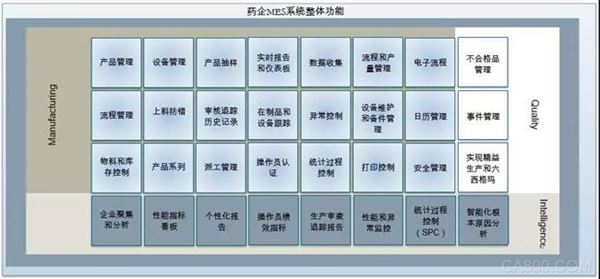 Compared with other industries, pharmaceutical companies have special characteristics such as complex process mechanism, many uncertain factors, strong production planning, cumbersome production scheduling management, and strict batch number management. In view of the special requirements of the MES system, it is necessary to effectively combine the enterprise system and operation mode, and implement it by reforming and reorganizing the production organization and management mode of the enterprise. After analysis, the main functional requirements of the production workshops of most pharmaceutical companies are work order management, process route management, material management, container management, production process control management, and field data collection.
Compared with other industries, pharmaceutical companies have special characteristics such as complex process mechanism, many uncertain factors, strong production planning, cumbersome production scheduling management, and strict batch number management. In view of the special requirements of the MES system, it is necessary to effectively combine the enterprise system and operation mode, and implement it by reforming and reorganizing the production organization and management mode of the enterprise. After analysis, the main functional requirements of the production workshops of most pharmaceutical companies are work order management, process route management, material management, container management, production process control management, and field data collection.
The specific instructions are as follows
01 Work Order Management
The MES system supports the manual creation of work orders and batch numbers based on the product name and output. At the same time, it can accept work orders containing product name, output, batch number and other information issued by the upper-level ERP system of the factory.
02 Process route management
The MES system specifies different process routes according to different products and different batches of information, and at the same time supports the optimization of existing process routes based on GMP and the company's existing production conditions.
03Material Management
GMP requires companies to establish a standardized material management system to make the material flow clear, controllable, and traceable. The MES system controls the input and output of materials by encoding the materials. After the encoding is generated, it is locked into the production process to effectively prevent the mixed batch of materials, and the material information is stored in the database through the encoding method, and the batch number, The electronic supervision code and other information are linked to facilitate users to trace back according to the product code information at any time.
04 Container Management
The final measurement of pharmaceutical preparations is based on the packaging of various preparations into containers. There are many types of containers in the MES system. In pharmaceutical companies, containers are mostly used for weighing modules. When the final sterilized products need to be sterilized, different batches will occur in the same cabinet. In order to prevent mixed batches, the sterilized vehicles will also be sterilized. They are treated as containers and are bound to different production batches for operation.
05Production process control management
The core content of the MES system is to improve the qualification rate of the company's products by effectively reminding the staff of the current operations that need to be performed, restricting the operating authority, controlling the key data of the production process, and controlling the key operations of the production process, and standardize the company's product data , The production process is standardized, so as to ensure that the company's product information is complete and standardized, and ultimately achieve the purpose of improving the quality of the company's products and enhancing the corporate image.
06 On-site data collection
The MES system collects the current operating data of the field equipment through the data acquisition system, avoiding the need for staff to manually confirm the data value and whether the value is accurate when filling in the record, and avoid the occurrence of errors as much as possible.
07 Electronic batch record
Electronic batch records are the current development direction of enterprises. The MES electronic batch recorder can display electronic instructions for the operator on the screen to ensure the integrity and accuracy of the batch files. Manual data entry is limited to situations that are absolutely necessary, and relevant inspections are always carried out in accordance with the requirements of the new version of GMP. If possible, we will collect data directly from the equipment and sensors to ensure the accuracy of the data. In any case, the integrated exception management function will manage deviations from the requirements of the process specification, which can significantly shorten the time required for batch inspections, and will also greatly reduce the workload of investigations related to deviations. Intelligent production and performance management tools can also provide advanced reporting and data analysis functions and dashboards. With the help of them, users can convert data collected during the production process into valuable information.
The MES system effectively integrates the enterprise management information system and the process control system, and integrates functions such as production data collection, transmission and processing, drug quality tracking and dynamic finished product control, online quality monitoring and management, and implementation of online optimization scheduling and cost management. Realize the whole process tracking of medicine manufacturing.
The specific approach of the vaccine sales side is to print the Chinese drug electronic supervision code (code) for each smallest unit of drug sales, from the manufacturer to the distributors at all levels, and then to the pharmacy. Code scanning records are required for verification and write-off to ensure that the drug can be traced throughout the entire process. The point of sale terminal will be equipped with a digital certificate (key) to query detailed information. Products that have not been connected to the Internet or have not used the unified identification of the drug electronic supervision code shall not be put on the market.
Vaccines are related to people's lives, health and safety, and there must be zero tolerance for any fraud. The reason for using PLM platform to manage drug R&D, MES system to record production process and monitoring quality, electronic supervision code to manage drug distribution and storage, to establish industrial big data and cloud for the entire life cycle of drug development, production records, quality monitoring, circulation, and storage On the platform, pharmaceutical companies have made such intricate regulations, precisely in order to force pharmaceutical companies to reduce the risk of uncertainty with compliant R&D, standardized production processes, and strict quality monitoring, and ultimately achieve controllable product quality... …Vaccines are related to the healthy growth that our country hopes for in the future, and the supervision of vaccines must be very strict rather than strict. The editor hopes that all kinds of vaccine manufacturers will learn from the lessons and strictly implement drug R&D, production, quality, and sales management practices. Resolutely put an end to fraud, cutting corners and other illegal activities.
Summary Lack of compliance research and development, imperfect electronic production records, imperfect quality control, materials, production processes, quality, raw materials cannot be traced, and other issues are not only the exclusive problems of the medical industry, but also in the food and beverage industry and the electronic high-tech industry. Many industries, such as new energy, chemical industry, national defense and military industry, also have many of the above problems. Tiantuo Sifang has been deeply engaged in digital factory solutions for fifteen years, providing enterprises with overall solutions for digital factories from product development, product design, process design, manufacturing, quality monitoring, automated production lines, automated warehousing and logistics, and the digital network nebula platform. Welcome to inquire!
Construction field, ships building industry, petroleum & chemical industries,
We have imported machine for protecting the quality of products.

How to eliminate falsified production records. Pharmaceutical companies first use the product lifecycle management (PLM) platform to ensure that drugs from R&D, pilot testing, clinical to registration applications must meet the compliance requirements of the State Food and Drug Administration (FDA); secondly, pass The Manufacturing Execution Management System (MES) system establishes a complete production process record in accordance with the Good Manufacturing Practices (GMP).
The production record of the pharmaceutical company must include: product name, specification, batch number, date and time of the start and end of production and intermediate processes, the signature of the person in charge of each production process, abnormal event reports and other data, using the real-time production collected by the MES system Record and strictly monitor the production process of medicines; the sales end of medicines should re-enable the electronic supervision code to strictly monitor the status of the production and circulation of medicines; finally, use the Internet of Things, industrial big data, and industrial cloud to ensure the development, manufacturing, and manufacturing of medicines. The entire process of quality monitoring, circulation, transportation, storage and distribution to medical institutions is under the supervision of the drug regulatory department to ensure that each box, each box, and each batch of key drug production records, operations, inventory and flow directions can be inquired in real time Happening.

The specific instructions are as follows
01 Work Order Management
The MES system supports the manual creation of work orders and batch numbers based on the product name and output. At the same time, it can accept work orders containing product name, output, batch number and other information issued by the upper-level ERP system of the factory.
02 Process route management
The MES system specifies different process routes according to different products and different batches of information, and at the same time supports the optimization of existing process routes based on GMP and the company's existing production conditions.
03Material Management
GMP requires companies to establish a standardized material management system to make the material flow clear, controllable, and traceable. The MES system controls the input and output of materials by encoding the materials. After the encoding is generated, it is locked into the production process to effectively prevent the mixed batch of materials, and the material information is stored in the database through the encoding method, and the batch number, The electronic supervision code and other information are linked to facilitate users to trace back according to the product code information at any time.
04 Container Management
The final measurement of pharmaceutical preparations is based on the packaging of various preparations into containers. There are many types of containers in the MES system. In pharmaceutical companies, containers are mostly used for weighing modules. When the final sterilized products need to be sterilized, different batches will occur in the same cabinet. In order to prevent mixed batches, the sterilized vehicles will also be sterilized. They are treated as containers and are bound to different production batches for operation.
05Production process control management
The core content of the MES system is to improve the qualification rate of the company's products by effectively reminding the staff of the current operations that need to be performed, restricting the operating authority, controlling the key data of the production process, and controlling the key operations of the production process, and standardize the company's product data , The production process is standardized, so as to ensure that the company's product information is complete and standardized, and ultimately achieve the purpose of improving the quality of the company's products and enhancing the corporate image.
06 On-site data collection
The MES system collects the current operating data of the field equipment through the data acquisition system, avoiding the need for staff to manually confirm the data value and whether the value is accurate when filling in the record, and avoid the occurrence of errors as much as possible.
07 Electronic batch record
Electronic batch records are the current development direction of enterprises. The MES electronic batch recorder can display electronic instructions for the operator on the screen to ensure the integrity and accuracy of the batch files. Manual data entry is limited to situations that are absolutely necessary, and relevant inspections are always carried out in accordance with the requirements of the new version of GMP. If possible, we will collect data directly from the equipment and sensors to ensure the accuracy of the data. In any case, the integrated exception management function will manage deviations from the requirements of the process specification, which can significantly shorten the time required for batch inspections, and will also greatly reduce the workload of investigations related to deviations. Intelligent production and performance management tools can also provide advanced reporting and data analysis functions and dashboards. With the help of them, users can convert data collected during the production process into valuable information.
The MES system effectively integrates the enterprise management information system and the process control system, and integrates functions such as production data collection, transmission and processing, drug quality tracking and dynamic finished product control, online quality monitoring and management, and implementation of online optimization scheduling and cost management. Realize the whole process tracking of medicine manufacturing.
The specific approach of the vaccine sales side is to print the Chinese drug electronic supervision code (code) for each smallest unit of drug sales, from the manufacturer to the distributors at all levels, and then to the pharmacy. Code scanning records are required for verification and write-off to ensure that the drug can be traced throughout the entire process. The point of sale terminal will be equipped with a digital certificate (key) to query detailed information. Products that have not been connected to the Internet or have not used the unified identification of the drug electronic supervision code shall not be put on the market.
Vaccines are related to people's lives, health and safety, and there must be zero tolerance for any fraud. The reason for using PLM platform to manage drug R&D, MES system to record production process and monitoring quality, electronic supervision code to manage drug distribution and storage, to establish industrial big data and cloud for the entire life cycle of drug development, production records, quality monitoring, circulation, and storage On the platform, pharmaceutical companies have made such intricate regulations, precisely in order to force pharmaceutical companies to reduce the risk of uncertainty with compliant R&D, standardized production processes, and strict quality monitoring, and ultimately achieve controllable product quality... …Vaccines are related to the healthy growth that our country hopes for in the future, and the supervision of vaccines must be very strict rather than strict. The editor hopes that all kinds of vaccine manufacturers will learn from the lessons and strictly implement drug R&D, production, quality, and sales management practices. Resolutely put an end to fraud, cutting corners and other illegal activities.
Summary Lack of compliance research and development, imperfect electronic production records, imperfect quality control, materials, production processes, quality, raw materials cannot be traced, and other issues are not only the exclusive problems of the medical industry, but also in the food and beverage industry and the electronic high-tech industry. Many industries, such as new energy, chemical industry, national defense and military industry, also have many of the above problems. Tiantuo Sifang has been deeply engaged in digital factory solutions for fifteen years, providing enterprises with overall solutions for digital factories from product development, product design, process design, manufacturing, quality monitoring, automated production lines, automated warehousing and logistics, and the digital network nebula platform. Welcome to inquire!
| About Copper Bar |
Construction field, ships building industry, petroleum & chemical industries,
war and electricity industries, food processing and medical industry, boiler heat exchange, machinery and hardware fields. Stainless
steel pipe can be made according to the customers requirements.
Standard export seaworthy or as per customers' requirement
|
Machine for Quality. |
We have imported machine for protecting the quality of products.

Copper Bar,Flat Copper Bar,Oxygen Free Copper Bar,Electrical Application Copper Bar Flat
HENAN HUAYANG ELECTRICAL TECHNOLOGY GROUP CO.,LTD , https://www.huaonwire.com
